Instruction
The AmniSure test is a swab only test with results produced within minutes. The test consists of 3 simple steps.
Call Us Today: (021) 66971814
Rapid, Reliable, Non-Invasive Test for Premature Rupture of Membranes (PROM)

AmniSure® solves a long-standing problem in obstetric practice - diagnosis of ruptured fetal membranes (ROM). Premature ROM (PROM) occurs in about one out of ten pregnant women and constitutes the major factor of pre-and post-natal complications. PROM occurs when a woman’s amniotic fluid breaks prematurely (not followed by labor in 24-48 hours). If the rupture of the amniotic sac that results in the leakage of the fluid is not detected and treated in a timely and accurate manner (during 24 hours since the rupture’s occurrence), infection and other serious complications for the neonate and the mother may occur.
AmniSure® detects the PAMG-1 protein in the amniotic fluid and allows accurate diagnosis so early intevention can occur.
AmniSure® is recommended by the RANZCOG guidelines.
Visit the AmniSure Website.
*according to published data
The AmniSure test is a swab only test with results produced within minutes. The test consists of 3 simple steps.
1 Vaginal Swab
1. Insert provided sterile collection swab 5-7cm deep into the vagina (no speculum needed).
2. Hold in place for 1 minute to ensure saturation.
3. Ensure that the provided sterile swab touches nothing prior to insertion.

2 Dilution
1. Insert swab into vial.
2. Rotate specimen in vial for 1 minute.
3. Dispose of swab. DO NOT break off swab and leave in vial.

3 Testing Sample
1. Insert test strip with arrows facing down within 30 minutes of sample collection.
2. Remove test strip from vial if two lines have appeared or after 5 minutes.
3. Place on dry flat surface to read. DO NOT read results after 10 minutes since dipping the test strip into the vial.

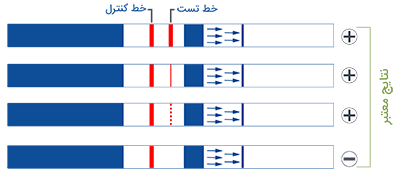
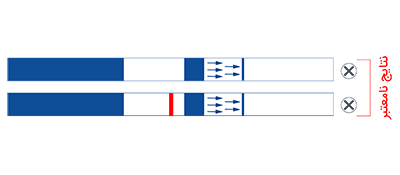

AmniSure® is a rapid test that aids in detecting Rupture Of [fetal] Membranes (ROM) in women with signs and symptoms suggestive of ROM. ROM presents the risks of infection, fetal distress, prolapse of the umbilical cord, postnatal endometritis and abruptio placenta.1,3 Premature ROM could also lead to premature delivery. All these consequences increase the likelihood of fetal and maternal morbidity and mortality. AmniSure® can help diagnose ROM in a timely and accurate manner, so that appropriate and opportune measures can be taken.
The test is for use by healthcare professionals (Rx only) to aid in the detection of fetal membranes rupture in pregnant women when they report signs, symptoms, or complaints suggestive of such a rupture. AmniSure® should be used in sites or by qualified personnel (physicians, certified nurse-midwives, or labor and delivery nurses certified to evaluate ROM) similar to those of clinical trials that were performed on the product. AmniSure® can therefore be used in a variety of settings, from OB/GYN clinics and doctors' offices to outpatient clinics and labor admitting rooms.
AmniSure® is a one-step immunochromatographic device. The test is based on the use of several, specifically selected, monoclonal antibodies that detect trace amounts of the amniotic fluid protein PAMG-1, which is present in cervico-vaginal discharge after the rupture of the fetal membranes. During the test procedure, PAMG-1 from the sample sequentially binds to a monoclonal antibody conjugated with the label particles, and then to antibodies immobilized on an insoluble carrier.
Placental alpha microglobulin-1 is a protein expressed by the cells of the decidual part of the placenta. During pregnancy, PAMG-1 is secreted into the amniotic fluid.
PAMG-1 was selected as a marker of fetal membranes rupture due to its extremely low background level in the presence of intact fetal membranes, when measured in cervico-vaginal discharge.
Based on published data, the AmniSure® test is ~99% accurate.1-5 In clinical trials, an AmniSure® test correlated with the clinical diagnosis obtained through the combined usage of three routinely used tests—nitrazine, ferning, and pooling. The simplicity of the test provides for equally accurate results when the test is conducted in OB/GYN clinics and exam rooms. The diagnostic accuracy of the AmniSure® test relies on its sensitivity threshold, which is set at the low level of 5 ng/ml (while the background cervico-vaginal concentration of PAMG-1 is only 0.05-0.22 ng/ml).
Whenever possible, the AmniSure® test should be performed immediately after sample collection. If necessary, however, samples can be stored in a refrigerator (at +4°C) for six hours.
In cases where trace amounts of blood are present on the swab, the test functions properly. When there is a significant presence of blood on the swab, the test can malfunction and its use is not recommended. In such cases, a separate sample without considerable amounts of blood should be taken and tested. The test result may be negative when the sample is taken 12 or more hours after a presumed fetal membrane rupture has occurred (i.e. due to a possible “resealed” rupture or the temporary obstruction of leakage).
Yes. AmniSure® should not be used within 6 hours after the removal of any disinfectant solutions or medications from the vagina.
A sealed AmniSure® test must be stored in a dry place, at 40°F to 75°F (+4°C to +24°C). The test should not be frozen or used beyond the expiration date stamped on the product. AmniSure® must be used within 6 hours after opening and the test kit components should not be reused.
AmniSure® is a one-step immunochromatographic assay. It detects trace amounts of PAMG-1, a protein found in amniotic fluid that appears in vaginal discharge after fetal membranes rupture. Monoclonal antibodies are used to detect the protein. AmniSure® works within a wide range of PAMG-1 concentrations potentially found in vaginal discharge (from 5 ng/ml to 100 μg/ml). The diagnostic accuracy of the test allows it to detect even a minuscule amount of released amniotic fluid. With intact fetal membranes, the test does not normally detect PAMG-1 due to its low background concentration.